Changing the paradigm for MVID
Vanessa Biotech will soon sponsor clinical trials of Shylicine™. To learn more about these trials, please contact us.
Our team at Vanessa Biotech is working on the first medicine that can treat microvillus inclusion disease, with the ultimate goal of eliminating the patient’s dependence on intravenous nutrition or TPN, and the need for a bowel transplant. These current treatment options limit a child’s quality of life and bring the risk of serious complications. With our research, we hope to change the way doctors and clinicians view the disease and prescribe treatment.
At the Yale School of Medicine, Dr. Dmitry Kravtsov identified the role that intestinal cell immaturity plays in the severity of diarrhea. His research suggested that the failure of the MVID intestine may not be permanent, and that the immature cells of the gut may be able to grow again. Armed with this understanding and combined with an exhaustive review of the literature from the past twenty-five years, our team has developed a cocktail drug formulation called Shylicine™. We envision Shylicine™ to be a treatment that does not require any form of surgery or medical procedure, and can be taken at home in a convenient oral form, without a medical professional or training.
The goal of our treatment is to:
- Help the intestine absorb water and nutrients,
- Reduce excessive secretion of ions and water,
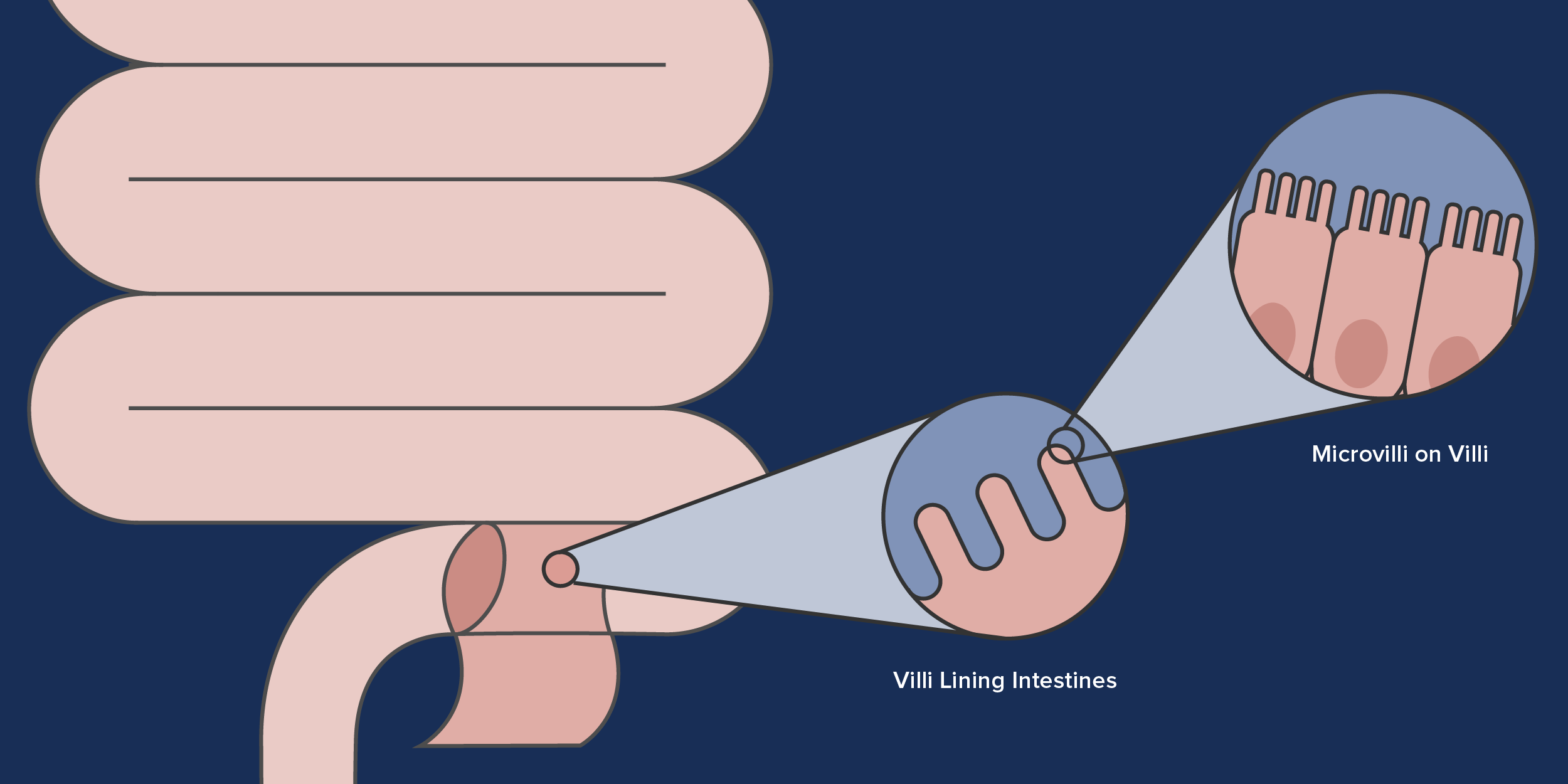
- Increase the absorptive surface of the intestine by restoring the villi and microvilli, and
- Maintain the required integrity of the intestinal barrier.